Small Molecules toward Grand Challenges
Rational Design of Modern Catalysts for the Production of Biobased Fine Chemicals
小分子奔向關鍵化學議題之路
生物質精緻化學品之現代催化劑的合理設計
The research interests of Dr. Chung’s group (CPWLab) are placed on three thematic topics: (1) the selective catalysis for C6 platform chemicals; (2) the controlled surface modification on metal oxides for C4 platform chemical production (ethanol upgrading); (3) the molecular understanding of C1+ platform chemical production from CO2 by investigating CO2 adsorption energetics. The experiments are mainly carried out in the Physical Inorganic Lab (B708) and the Inorganic Material Lab (B712).
(1) Selective Catalysis for C6 Platform Chemicals
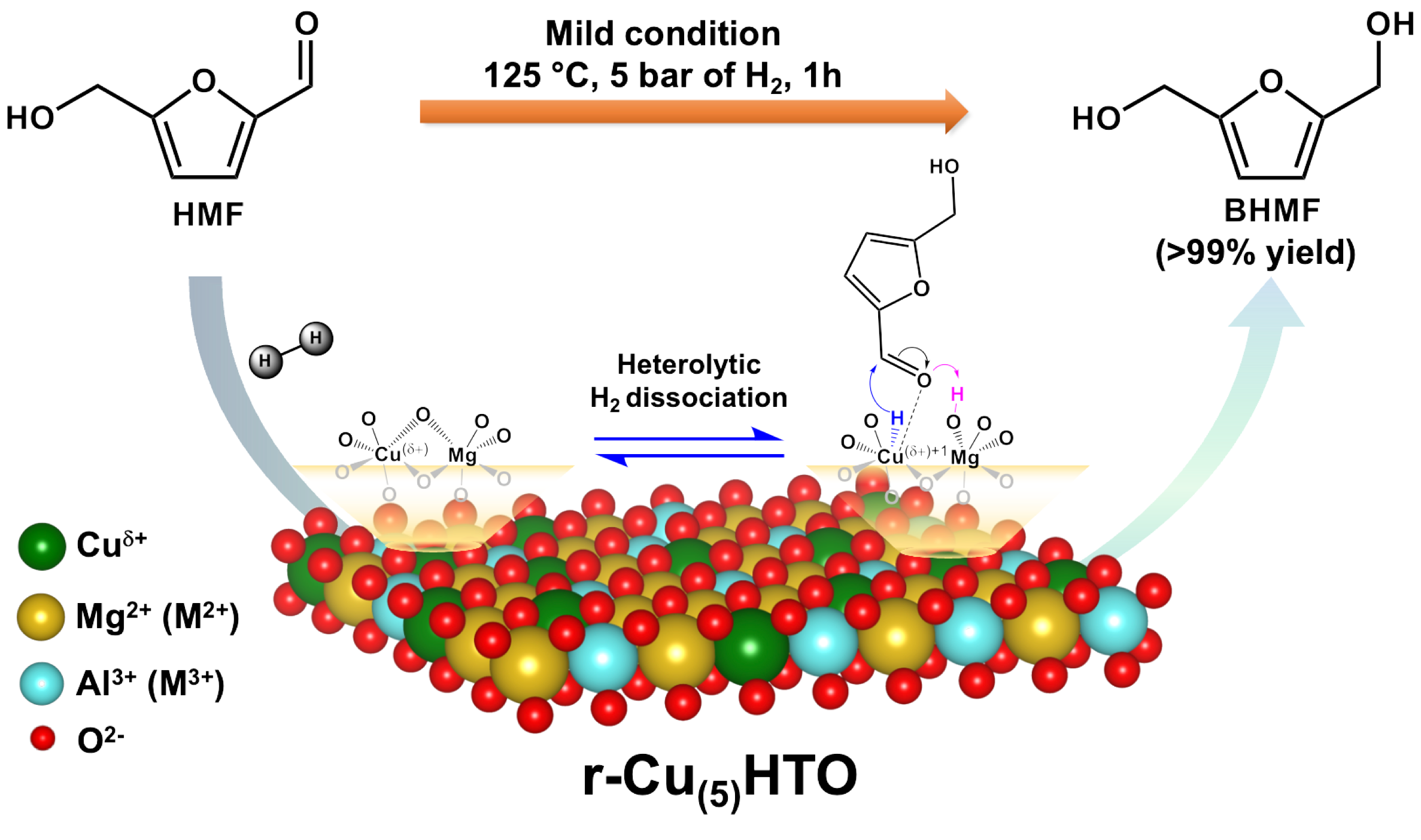
Lignocellulosic biomass has been extensively investigated as a renewable and inedible feedstock for the production of bio-based chemicals and liquefied fuels, especially while the UAE Consensus calls on Parties to transition away from fossil fuels to reach net zero after COP 28. Among those biomass-derived chemicals, 5-hydroxymethylfurfural (HMF) is regarded as on primary molecular building block for the further synthesis of value-added products, including 2,5-dimethylfuran (DMF), 2,5-Bis(hydroxymethyl)furan (BHMF), 2,5-dimethyltetrahydrofuran (DMTHF), 2,5-furandicarboxylic acid (FDCA), levulinic acid, 1,6-hexanediol, and long chain alkanes, etc. See Scheme 1 for the illustration.
Given the fact that the aforementioned C6 furanic chemicals are structurally analogous to those derived from petroleum industry, they have a wide range of industrial applications for manufacturing commodity products. In addition, recent arising of the shale gas enables the cheap and massive ethylene production derived from the dehydrogenation of ethane, which yields little butadiene (BD) production. Thus, it minimizes the necessity of the petroleum-based routes to produce BD, and it navigates the research direction towards the biobased process for C4 chemicals since the global production of bioethanol derived from lignocellulosic biomass has been growing continuously.
(2) Controlled Surface Modification on Metal Oxides for C4 Platform Chemical Production (Ethanol Upgrading)
Chung's research group has recently demonstrated that the surface nature of nanoscale hydrotalcite-derived oxide (HTO) can be simply tailored through the addition of amorphous silica during the coprecipitation process of material preparation. The change in physical/chemical properties of the modified surface has been carefully scrutinized by a series of surface characterization techniques The hallmark of this study is to hypothesize that the silanol groups on silica might serve as surface anchors to preferentially graft “(IV)-to-(IV)”-coordinated interface between HTO and silica surfaces during the preparation of materials, especially for the strong Si(IV)–Al(IV) connectivity. The first deposited aluminum (Al) species on silica in HTO/SiO2-X could result in Al(IV) tetrahedrally coordinated site (Al3+tetra) as shown in Scheme 2.
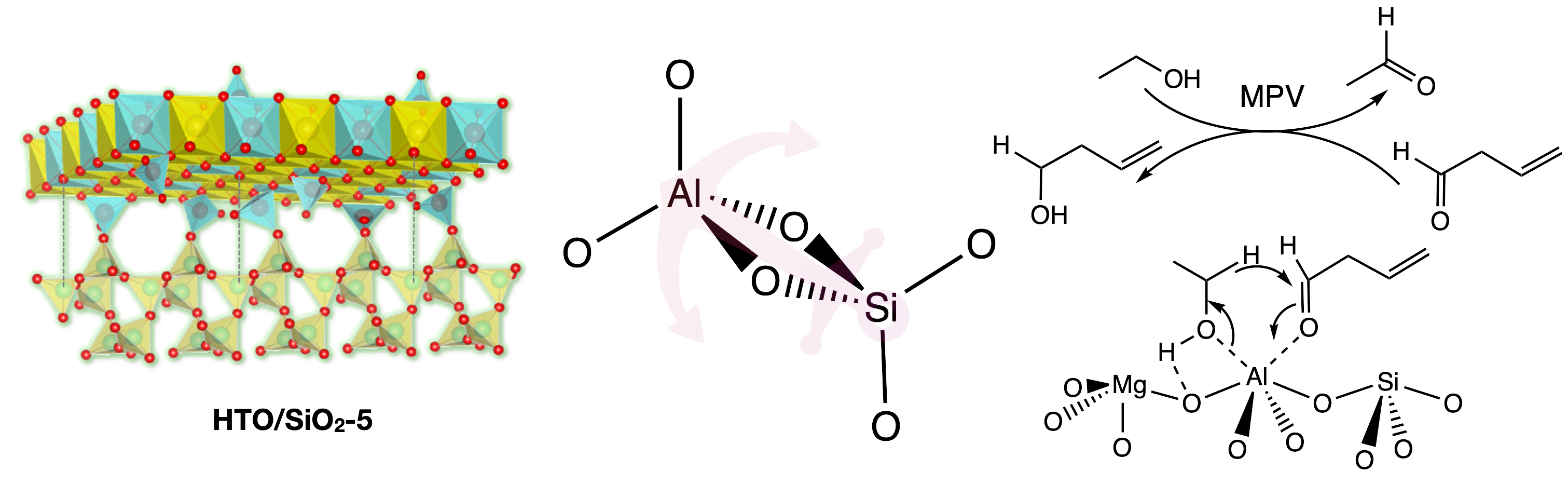
This specific surface site connectivity enables the formation of the newly-formed ternary magnesium-aluminum-silicon (Mg-Al-Si) interface with abundant tetrahedrally coordinated (Al3+tetra) sites, which has been comprehensively revealed and discussed in the SSNMR section (ACS Applied Nano Materials 2022, 5 (6), 7885–7895). As a result, the silica-supported HTO (i.e., HTO/SiO2-X) could catalyze ethanol condensation to foster C4 chemicals at 250 ℃ through the flow reactor. The HTO with a strong basic nature promoted 1-butanol production (66% selectivity), while HTO/SiO2-5 with more well-distributed acidic sites favored 1,3-butadiene production (43% selectivity). Hence, the surface modification could not only enhance the ethanol affinity on the surface of HTO/SiO2-5, but also facilitate the feasible formation of the six-membered cyclic intermediate centered by active sites with adsorbed crotonaldehyde and ethanol for the Meerwein-Ponndorf-Verley (MPV) reduction, which leads to the 1,3-butadiene production selectively from ethanol condensation.
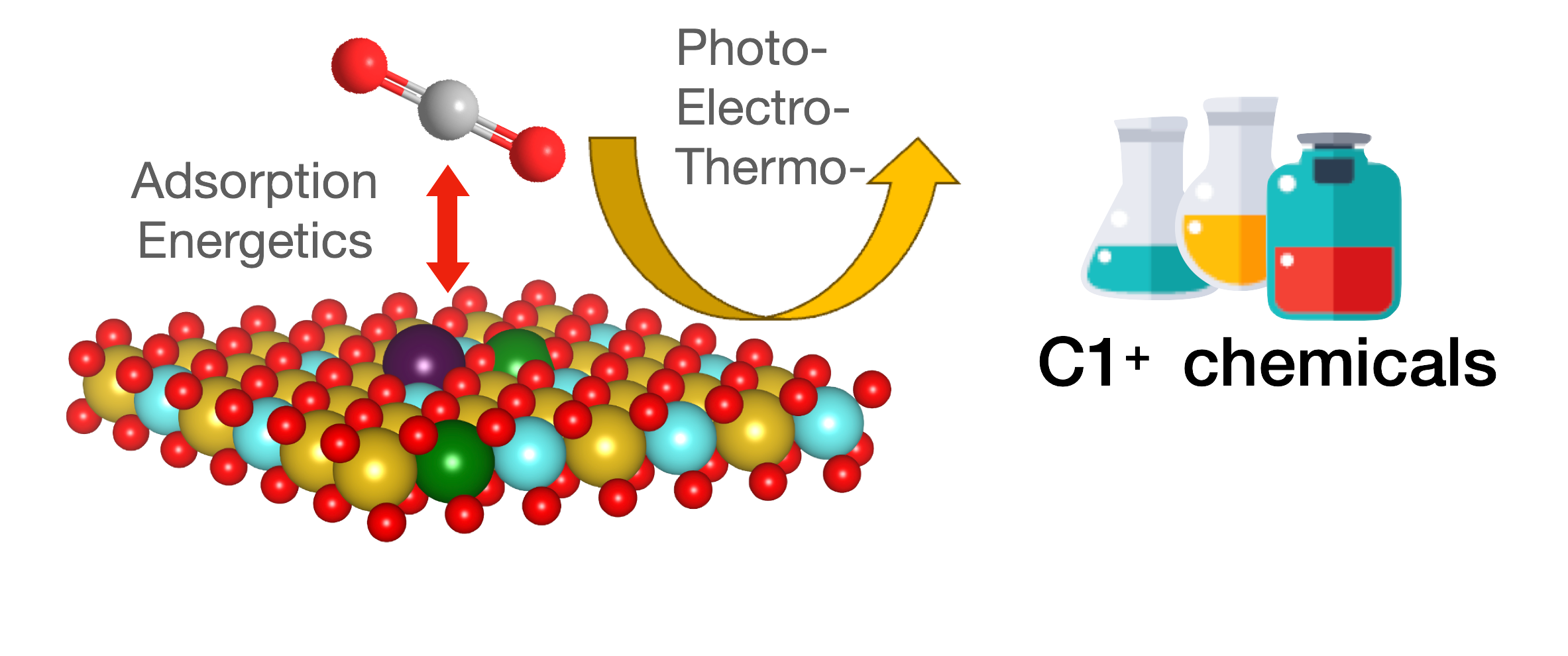
(3) Molecular Understanding of C1+ Platform Chemical Production from CO2 by Investigating CO2 Adsorption Energetics
Photocatalytic carbon dioxide (CO2) reduction reactions (CO2RR) have attracted significant attention in recent years as half a cycle of the artificial photosynthesis. To understand the CO2RR adsorption energetics on the photocatalyst surface, Prof. Chung and coworkers have revealed textural information on the catalyst surface by performing the CO2 adsorption study. Interestingly, the catalytic performance of photocatalysts for CO2 reduction is highly correlated to the CO2 adsorption energetics in different catalytic systems. In other words, photocatalysts for CO2RR with better efficiency can provide more active sites and different surface energy exposed for CO2RR adsorption.